Case Studies
Explore our case studies highlighting groundbreaking research powered by data in the National Genomic Research Library.

Unlocking heart genetics: Age and sex influence on cardiomyopathy
This study by Akinrinade and colleagues explores how genetic factors contribute to cardiomyopathy; a heart condition where muscles of the heart do not function normally. The research investigates how genetic variants impact the development and progression of this condition, focusing on how this differs across age groups and between sexes. Understanding this interplay between genetics, age, and sex offers insight into the condition's development, potentially leading to more effective strategies for risk assessment and treatment. Ultimately, this research highlights the importance of tailored, personalised interventions to diagnosing and treating cardiomyopathy, based on individual characteristics.

Revisiting undiagnosed conditions using new genomic advances
This study by Moore and colleagues uses newer, more advanced approaches to reanalyse data from the 100,000 Genomes Project, helping to find answers for participants. Lots of families in the rare disease arm of the 100,000 Genomes Project have rare conditions with a suspected genetic cause. However, many of these families are still without a concrete genetic diagnosis. Recent advances in the way we analyse whole genome sequencing data have greatly improved our ability to solve these more complicated diagnostic cases. Using these new methods, this study reanalysed data from 31 undiagnosed families, and found a genetic diagnosis for 8 of them. Receiving this genetic diagnosis can be lifechanging for participants, opening doors for potential treatments and providing answers for them and their families.
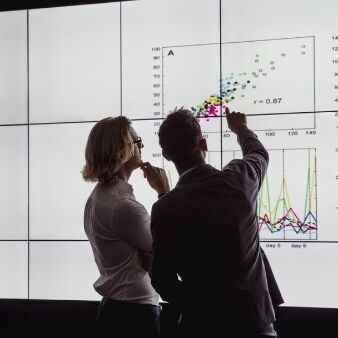
A better chance of diagnosis and treatment for pulmonary lung disease
Through engagement with the Genomics England Research Network, the team at Boehringer Ingelheim collaborated with leading academics in the progressive fibrosing interstitial lung disease (PF-ILD) area, as well as transcriptomics and proteomics providers and analysts to develop groundbreaking multiomic research in this field. Access to the National Genomic Research Library allowed the team to analyse genomic data from patients with evidence of PF-ILD. They generated new insights on multiomic data, and built RNAseq and proteomics on top of the genomic and clinical data for this cohort. Furthermore, their research will assess the similarities and differences in the underlying genetic mechanisms driving this disease, and correlating these with people who were found to be severely affected by COVID-19.
These studies demonstrate the breadth of opportunities that are possible when partnering with Genomics England. The analysis should reveal new and improved multiomic characterisation and substratification of PF-ILD, helping to improve the accuracy of diagnoses. This will enable more appropriate recruitment criteria for clinical trials, and enhance research and development in this therapeutic area.

Creating a platform for an oncology therapy portfolio prioritisation
Given the phenotypic diversity of a cancer tumour type, a large pharma company needed more genetic insights to prioritise potential therapies to target the disease area.
Genomics England leveraged the 100,000 Genomes Project data, as well as its network of clinical experts, to assemble the specific clinical and outcomes data needed in a bespoke cohort exclusive to the partner company.
The pharma company now has access, through the Genomics England platform, to a bespoke dataset to guide drug development in oncology. Initial outputs include a landscape research paper published in peer review journals.
Framing the genetic basis of a disease to identify druggable targets and accelerate commercial sales
A mid-sized rare disease focused biopharma company had an anti-sense oligonucleotide (ASO) therapy targeting a rare disease. Given the limited understanding of the genetic basis of the disease, they needed a stratified data model to more effectively identify druggable targets, recruit for trials and drive commercial success.
Focusing on particular variants associated with the rare disease that were considered to be pathogenic, Genomics England collaborated with this company to explore the 100,000 Genomes Project and found one of these variants to be present in affected, as well as unaffected individuals.
With this finding as the promising beginning of the genetic basis of the disease, the biopharma company commissioned the follow-on work to complete the stratification model and genetic framework to guide their rare disease business going forward.
