Scientists find new genetic causes of sight loss that could provide answers for thousands of patients
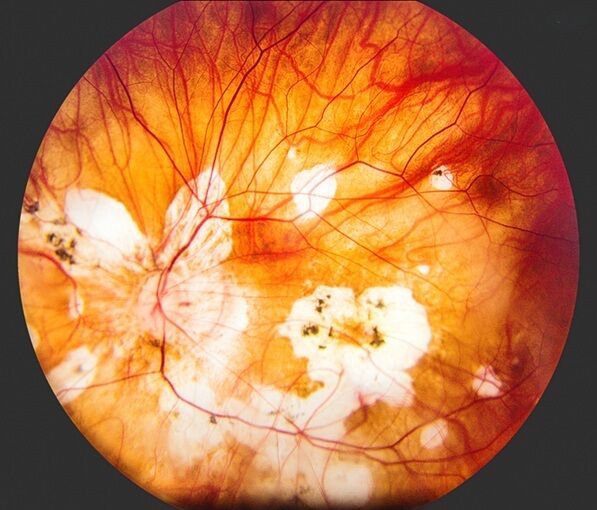
Scientists analysing data from Genomics England’s National Genomic Research Library (NGRL) among other sources have uncovered five new genetic causes of retinitis pigmentosa (RP), an eye condition that causes gradual blindness. The researchers estimate that the discovery could provide a genetic diagnosis for thousands of people for the first time, with over 150 individuals already having received diagnoses.
The research, led by the University of Basel and Radboud University Medical Center and published in Nature Genetics, looked at the DNA and health data of over 4,700 individuals with RP. It found that changes in the RNU4-2 gene and changes in four RNU6 genes are linked to the condition. The researchers estimate these changes could provide answers for around 1.4% of unsolved RP cases around the world.
RP is the most common cause of inherited sight loss and approximately two million people worldwide are affected by it. It is estimated that 30% to 50% remain without a conclusive genetic diagnosis, even after genome sequencing is performed. Many different genes are linked to the condition, although scientists still don’t fully understand why problems in these genes cause damage to the retina in the way they do.
The RNU4-2 gene has already previously been linked to ReNU syndrome – a neurodevelopmental condition that was also discovered in 2024 through access to data held by Genomics England in the NGRL. ReNU syndrome commonly affects learning, speech, movement and behaviour. Previous studies have also shown that more than half of patients with ReNU also had some vision problems.
This research showed that the RNU4-2 gene can lead to different genetic conditions depending on the specific change within the gene and where in the gene the change happens – some changes cause neurodevelopmental disorders such as ReNU syndrome, while others (in a different region of the RNU4-2 gene) lead to RP and gradual sight loss.
RNU4-2 and RNU6 genes both create pieces of RNA that combine together, and with other genes, to help ensure that other genes can be ‘switched on’ and operate correctly. But when genetic changes impact RNU4-2 or the affected RNU6 genes in a way that stops them from fitting together, the retinal cells struggle to function over time and eventually stop working as well. This breakdown in the retina is what causes the vision problems associated with RP.
“The changes that have been uncovered through this research could provide a genetic diagnosis for thousands of patients affected by genetic sight loss for the first time. The discovery will help improve both the ability to diagnose it as well as genetic counselling, which can be a very helpful resource for many people with a rare condition.
“The genetic changes described for the first time in this study through whole genome sequencing data expand our understanding of the genetic basis of visual impairment and can now be integrated into standard genetic diagnostic testing. The genes that have been uncovered are non-coding RNA genes - meaning they don’t make proteins. It’s more difficult to understand what effect changes in these types of genes have, and these findings increase evidence for the importance of non-coding RNA gene research.
“These findings also provide a new avenue for research into developing treatments and genetic targets to focus on.”
Jamie Ellingford
Lead genomic data scientist at Genomics England
“We’ve learned that changes in these RNA genes can be just as impactful as changes in protein-coding genes. This is fundamental knowledge that broadens our understanding of hereditary diseases.”
Susanne Roosing
Molecular geneticist at Radboud and lead researcher
“What makes this discovery particularly intriguing is that it involves one of the most fundamental cellular processes, RNA splicing, yet the disease manifests almost exclusively in the retina. This shows how even subtle disturbances in core biological machinery can have highly specific effects in vulnerable tissues like photoreceptors.”
Carlo Rivolta
Head of the Ophthalmic Genetics Group at the Institute of Molecular and Clinical Ophthalmology Basel


